Which subatomic particle determines an atom's atomic number? A student proposed the "plum pudding" model of an atom. The student's model is different from the modern models because modern models show thatWhich of the subatomic particles has a negative charge?a subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. A property of an atom which increases with its tendency to attract the electrons of a bond. Atoms. Hydrophillic. having a tendency to mix with...What particle has a charge of? An atom is composed of three types of subatomic particles: electrons, protons and neutrons. Most subatomic particles have either a positive (+) or negative (−) electrical charge. Those that don't are considered neutral.Standard Model of particle physics. Elementary particles of the Standard Model. v. t. e. In physical sciences, subatomic particles are smaller than atoms.This preview shows page 4 - 6 out of 7 pages. 3.Which subatomic particle has a negative charge? 8.Which electron energy level transition corresponds to a hydrogen atom absorbing a visible light photon that has a wavelength of 656 nanometers?
a stable subatomic particle with a charge of negative electr
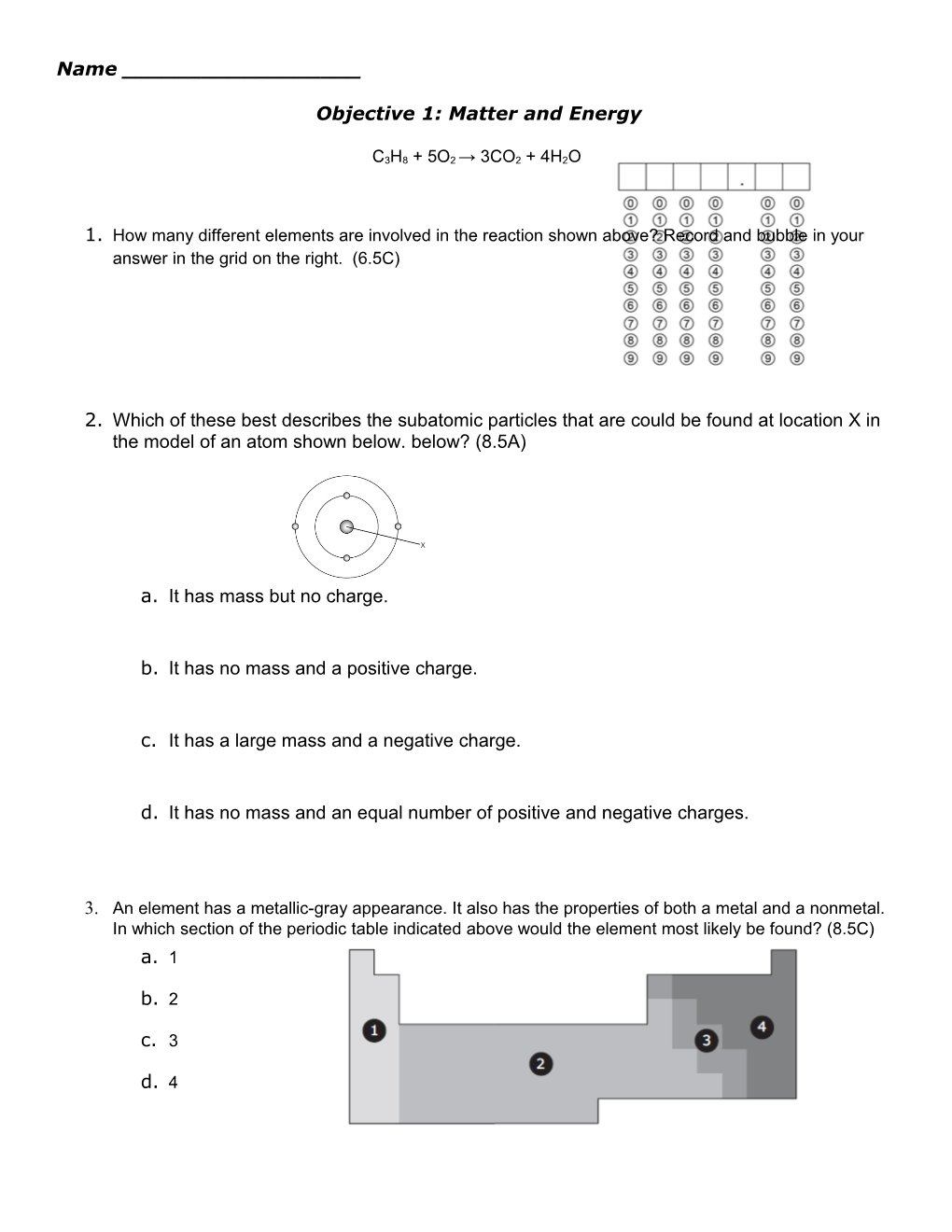
Subatomic particles are the most elementary particles found in nature. The three basic subatomic particles that comprise an atom are protons, electrons The electron's mass is considered negligible and the elementary charge (negative) and angular momentum are constant. The electron has a...Which subatomic particles has no charge? Neutron. What subatomic particle has the greatest mass? Electron cloud. Where in the atom will the electrons be found? When the number of electrons is greater than the number of protons. When will an ion have a negative charge?which subatomic particle has a negative charge? Answer: Electron. Explanation: There are three subatomic particles. out of all electron is negatively charged, proton is positively charged while neutron is a neutral particle. Question 2: if an atom has 2 protons, 3 neutrons, and 3 electrons, what...Subatomic particle - Subatomic particle - Antiparticles: Two years after the work of Goudsmit and The negative solution was more of a mystery; it seemed to describe electrons with positive rather than Positrons are very much like electrons: they have the same mass and the same spin, but they...
Which subatomic particles have a negative charge? | AnswersDrive
Discovery of Subatomic Particles. Sehat Nauli. A Crash Course In Particle Physics (1 of 2). powerphyzix.Electron has a negative charge. An atom is made up of nucleons and the electrons. There are several electrically neutral (in physics a neutral particle has no electric charge) subatomic particles. The most common ones include photons, neutrons and neutrinos.Electrons are negatively charged subatomic particles that are as negative as protons are positive. In general, atoms like to have the same number of electrons as they have protons in order to be electrically balanced. Electrons also play an important role in how atoms bond to each other to form...A particles's charge is not a measure of the particles's energy content. Instead, the definition is I found this definition/description a bit lacking, and I still don't grasp the nature of a "subatomic The positive and negative charges are just by definitions: if we wanted, we could define protons to be...— Outline Only —This article is only a brief description of the subject and is not intended to give a full explanation. Check out the "see also" or "references" sections, or Wikipedia's article for more detail. Subatomic particles are things smaller than an atom.
Yahoo has made the verdict to shut down Yahoo Answers. To better lend a hand you with this transition we now have compiled a listing of questions that may come up for you throughout this procedure.
What's the timeline for this procedure? April 20th - You may not be capable of publish new Yahoo Answers questions or resolution different users' questions.May 4th - The web page would possibly not be obtainable. If you try to get admission to Yahoo Answers on May 4th you'll be redirected to the Yahoo homepage. Will this affect my Yahoo Account or other Yahoo services?No, these adjustments are explicit to Yahoo Answers. They may not have an effect on your Yahoo Account or any other Yahoo products and services.
Where must I'm going when I have questions at some point?Visit Yahoo Search for answers and data from the internet. For specific info and sources across the Coronavirus pandemic, please discuss with our Yahoo COVID web page.
Can I obtain my Yahoo Answers content? What content is to be had to me?Your Yahoo Answers knowledge download will go back all user-generated content together with your Questions record, Questions, Answers checklist, Answers, and any photographs. You will be unable to download other users' content material, questions, or answers.
Do I have to download my content material?No, the content material download is optional. However, if you choose to download your Answers content, you should accomplish that prior to June 30, 2021.
When will I get my Yahoo Answers content?Our crew works as rapid as conceivable to make information to be had, but it might take as much as 30 days to obtain your content obtain.
I downloaded my Yahoo Answers content material, how do I view it?Your content material might be formatted in JSON (JavaScript Object Notation) and may well be arduous to look through at a look. Our article on viewing and managing your account knowledge allow you to perceive your knowledge download.
How can I proportion my feedback/feedback about this alteration?Send any feedback or feedback you will have referring to this determination to yahoo_answers_sunset@verizonmedia.com. Thanks for taking the time to percentage your ideas with us.
Solved: 12. Match The Subatomic Particles To Each Of The D... | Chegg.com

List The 3 Main Types Of Subatomic Particles And Indicate The Mass And Electrical Charge Of Each.
Which Atomic Particle Has A Negative... | Trivia Answers | QuizzClub

List The 3 Main Types Of Subatomic Particles And Indicate The Mass And Electrical Charge Of Each.
The Structure Of The Atom
SOLVED:An Alpha Particle Can Be Produced In Certa…

What Is A Very Small Particle Called

KEY - Practice MC And Short Answer

Lakhmir Singh Chemistry Class 9 Solutions For Chapter 4 Structure Of Atom - Free PDF

Quarterly 1 Review Trupia - Trupia

Objective 1: Matter And Energy - Docest
How Particle Accelerators Work | Department Of Energy

Solved: Which Subatomic Particle Has A Negative Charge? A.... | Chegg.com
Ns Ie Tound In C?C?1C? Q2. How Many Neutrons Are Found In 12C? 13C?"C? 12 C13... - HomeworkLib

Chapter 4: The Structure Of The Atom
Amazon.com: The Electron (Library Of Subatomic Particles) (9780823945283): Bortz, Fred: Books

Solved: 20. A Ca?* Ion Differs From A Ca Atom In That The | Chegg.com

Atoms And The Periodic Table V=pO0X6fVre1I&feature=related V=pO0X6fVre1I&feature=related - Ppt Download

Sample Pages - Mike Holt Enterprises

Exploring Atoms

What Is Effective Nuclear Charge?

0 comments:
Post a Comment